Cardiology practices face growing challenges in managing adverse event reporting, especially with Cardiac Implantable Electronic Devices (CIEDs) and chronic diseases. Outdated systems often lead to fragmented data, manual errors, and missed opportunities for better patient care and revenue. This guide explores how modern, integrated platforms like Rhythm360 can help address these issues, improving patient safety, streamlining workflows, and enhancing financial outcomes.
Many cardiology practices rely on outdated systems for adverse event reporting, creating risks for patients and inefficiencies for staff. Let's break down the core problems hindering effective management.
Cardiology practices often deal with devices from various manufacturers like Medtronic, Boston Scientific, Abbott, and Biotronik. Each uses its own portal for data access, leading to scattered information. This makes it hard to get a complete picture of a patient's device status or quickly spot issues, slowing down comprehensive adverse event reporting and follow-up as noted in industry guidelines.
Entering data by hand, transcribing records, and reconciling information across systems create a heavy workload for staff. These tasks are prone to mistakes, raising the chances of missing critical events and contributing to clinician burnout. Such inefficiencies pull focus away from patient care, going against efforts to lessen administrative burdens.
Without a centralized system to manage alerts, serious issues like atrial fibrillation, ventricular tachycardia, or device failures can slip through. Unreliable data or too many notifications cause alert fatigue, making it easy to overlook urgent problems that need immediate attention.
Not having a unified way to track and document billable services related to adverse events often results in lost revenue and denied claims. This is especially true for complex remote monitoring CPT codes, which are vital for a practice's financial stability.
Many current systems only react to events after they happen, missing the chance to use data for predicting and preventing issues. There’s a clear need for stronger internal reporting and follow-up processes to stop problems before they start, as highlighted by expert recommendations.
Rhythm360 offers a cloud-based platform that tackles key issues in adverse event reporting by unifying data and automating processes for CIEDs and chronic condition monitoring.
This solution brings scattered data into one place, making patient monitoring and event management more effective. It cuts down on paperwork, helps spot critical issues faster, and supports accurate billing documentation for better revenue. Schedule a demo to see how Rhythm360 can help your practice.
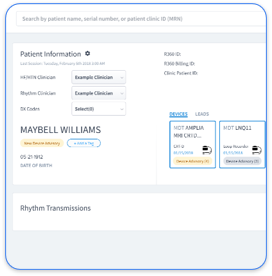
Rhythm360 pulls data from all major CIED manufacturers into a single view, removing the hassle of juggling multiple portals. This consolidated record is key for efficient monitoring and quick detection of adverse events.
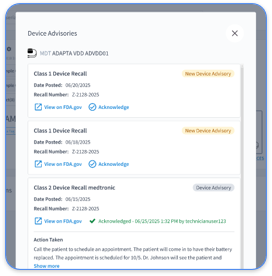
With AI and advanced data tools, Rhythm360 ensures over 99.9% accuracy in data delivery. It filters out unimportant alerts, focuses on significant issues, and can cut response times for urgent alerts by up to 80%, helping staff act quickly.
The platform handles report creation and documentation for billing and compliance needs. This eases administrative tasks and helps practices recover revenue while keeping clear records of adverse events.
Rhythm360’s communication tools allow automated or manual patient messaging, all tracked within the patient record. This ensures timely follow-up for potential issues or abnormal readings.
A secure, HIPAA-compliant mobile app lets clinicians check data, sign reports, and coordinate care from anywhere, supporting constant oversight of patient safety and event risks.
Adverse event reporting for CIEDs has changed with new technology, stricter regulations, and a focus on protecting patients. Here's how the field is advancing.
In the past, reporting focused on documenting events after they occurred. Now, with remote monitoring and AI, systems can catch early signs of trouble in device performance or patient health, enabling action before issues escalate.
Groups like the FDA and Heart Rhythm Society push for better reporting standards. Requirements like the Unique Device Identification System and Medical Device Reporting rules under 21 CFR Part 803 highlight the need for reliable systems. Programs such as the VHA's National Cardiac Device Surveillance Program show a structured way to manage device alerts.
Multiple CIED makers mean a maze of separate data systems. Vendor-neutral platforms that combine all data into one view are gaining ground, aiding clinical decisions and simplifying event reporting.
AI and machine learning help spot patterns and predict device issues, cutting through alert clutter to focus on urgent matters and support faster intervention as supported by recent findings.
Linking adverse event data directly to electronic health records prevents manual errors and keeps a full patient history. Automating these workflows also lightens the load on staff, aligning with industry efforts to improve efficiency.
Modernizing adverse event reporting takes careful planning to ensure it fits your practice's needs and delivers real value.
Practices must consider whether to create a custom system or adopt an existing platform. Given the complexity of compliance requirements like 21 CFR Part 803 and UDI rules, choosing a ready-made, vendor-neutral solution like Rhythm360 often saves time, money, and reduces risks.
Switching systems isn’t just about technology, it’s about people too. Evaluate your IT setup, staff training needs, and how to support change within your team. Open communication and thorough training are essential to ease concerns and ensure adoption.
Look beyond initial costs to long-term gains like better patient safety, faster alert responses, compliance with rules, and increased billing accuracy. Set clear goals, such as reducing alert response times or boosting revenue from remote monitoring services.
Handling sensitive patient information means prioritizing security and HIPAA compliance. Choose a system with strong encryption, secure access, and audit capabilities to protect data and avoid breaches.
Many cardiology practices are moving to integrated platforms for adverse event reporting. Rhythm360 stands out by unifying data and improving workflow efficiency.
Rhythm360 combines CIED and remote patient monitoring data into a single system, unlike older setups with separate portals. This makes monitoring and spotting potential events easier and more effective.
Using AI, Rhythm360 prioritizes alerts and cuts response times for urgent issues by up to 80%. Clinicians can focus on what matters most, addressing events promptly.
The platform automates documentation for billing and compliance, helping meet regulatory demands while ensuring accurate capture of billable services for improved revenue.
With real-time data, smart alerts, and built-in communication, Rhythm360 enables proactive care. Practices can address potential issues early through timely follow-ups or adjustments.
Before implementing a new adverse event reporting system, cardiology practices should evaluate their current setup and plan for a smooth transition. Use this framework to guide your process.
Start by understanding where you stand with data, workflows, and compliance.
Engage the right people to support the change.
Ensure you have the budget, time, and training plans in place.
Think about how the new system connects with existing tools.
Define ways to measure the system's impact, including:
Category | Ad-Hoc/Manual (Level 1) | Partially Integrated (Level 2) | Optimized/Proactive (Level 3) |
Data Sources | Multiple OEM portals, manual entry | Some automated feeds, still silos | All CIED/RPM data in one platform |
Alert Triage | Manual review, high fatigue | Basic filtering, slow response | AI-driven, prioritized alerts |
Reporting | Manual, error-prone tasks | Some automation | Fully automated, compliant |
Efficiency | High burden, frequent rework | Modest gains | Significant time savings |
By reviewing these areas, practices can plan a strategic move to a modern reporting system that supports clinical, operational, and financial goals. Schedule a demo to explore how Rhythm360 fits your needs.
Even experienced cardiology teams can run into problems when updating adverse event reporting. Avoid these pitfalls to ensure success.
Some teams stick to separate manufacturer portals, thinking small fixes are enough. But without a unified platform, data remains scattered, limiting a full view of patient risks and slowing event detection across devices, as emphasized in updated CIED guidelines.
Meeting basic regulatory needs, like FDA reporting rules or Medicare standards, isn’t enough. This narrow focus misses chances to improve operations, patient safety, and revenue with a fully integrated system.
Some clinicians doubt AI, sticking to old methods. Yet, ignoring AI’s ability to filter alerts and highlight urgent issues can lead to ongoing fatigue and delayed action, impacting outcomes. The VHA’s approach to quick alert review shows the value of efficient tools.
Focusing just on technology without preparing staff for change often backfires. Without proper training and clear benefits, even the best system faces resistance, leading to the same inefficiencies it was meant to fix.
Viewing a new system as just an expense, rather than a long-term gain, is a mistake. Modern tools save staff time, speed up critical responses, and improve billing accuracy, offering substantial returns over time.
A platform like Rhythm360 brings data from all CIED manufacturers into one place. This cuts the need to check separate portals, reducing errors and workload. Clinicians get a full, real-time view of patient devices, aiding faster event detection and reporting.
Manual review of alerts often leads to overload and missed issues. Rhythm360 uses AI to sort alerts, focus on critical events, and reduce response times by up to 80%. This prioritizes vital information for better patient safety.
Platforms like Rhythm360 automate data handling and billing documentation, cutting overhead costs. By capturing billable remote monitoring services accurately, practices can see revenue gains, with some increasing profitability significantly.
Key rules include the FDA’s Medical Device Reporting under 21 CFR Part 803 and Unique Device Identification System for tracking. Medicare also requires internal event reporting. Rhythm360 automates documentation and keeps auditable records to help meet these standards.
When choosing a system, focus on compatibility with all CIED makers, AI for alert prioritization, EHR integration, strong security for HIPAA compliance, automated compliance tools, and proven financial benefits. Good training and support during rollout are also critical.
Cardiology practices managing CIEDs and chronic conditions need advanced adverse event reporting to keep up with demands. Sticking to fragmented systems and manual tasks may not support patient safety or financial health. Rhythm360 provides a unified, AI-driven platform to improve how events are detected and managed.
Discover better patient safety and revenue potential. Schedule a demo of Rhythm360 to learn how it can enhance your reporting and workflows.
Ready to improve safety and efficiency with a modern system? Schedule a Rhythm360 demo now to connect with an expert and see how it meets your practice’s needs.