Navigating healthcare regulations for Cardiac Implantable Electronic Devices (CIEDs) and Remote Patient Monitoring (RPM) has become a core focus for cardiology practices. These rules go beyond patient privacy, requiring a firm grasp of billing codes, data security, and anti-kickback laws to maintain financial stability. This guide offers a clear path to understand and manage compliance, turning challenges into opportunities for better revenue cycle management (RCM) with practical strategies and automated tools.
For practices handling numerous patients with implanted devices, the risks are significant. A single compliance error can lead to hefty fines, exclusion from federal programs, and lasting damage to reputation. On the other hand, mastering these regulations can help recover lost revenue, improve operations, and enhance patient care through better monitoring.
Cardiology practices operate under a complex set of federal rules that cover patient data and physician referrals. Three key laws shape compliance, each with specific requirements that impact billing and revenue management for cardiac devices.
Safeguarding Cardiac Data with HIPAA
For practices managing CIED and RPM data, HIPAA compliance involves more than protecting patient privacy. The challenge comes from handling data across various sources like manufacturer portals, EHR systems, and mobile apps. Every point of access needs robust security to meet federal standards.
Modern cardiac monitoring produces vast amounts of sensitive health information flowing between patients, devices, servers, providers, and billing systems. Practices must use strong encryption, access limits, and detailed logs to protect this data while supporting real-time decisions for patient care.
Stark Law and Referral Rules
Stark Law sets strict rules on physician referrals, with fines up to $15,000 per violation and $100,000 for schemes to bypass it. Intent doesn’t matter; a violation happens even if it’s accidental.
In cardiology, this law affects choices of devices, monitoring services, and revenue agreements with tech providers. Working with multiple manufacturers or third-party services adds complexity, as each deal must avoid financial ties that could look like payment for referrals.
Anti-Kickback Statute and Financial Incentives
The Anti-Kickback Statute bans offering or accepting payment to encourage referrals for federal healthcare programs, with fines up to $100,000 and prison terms up to ten years. Unlike Stark Law, intent matters here, but knowledge of the law isn’t required for penalties since the Affordable Care Act.
Violations under this law can lead to severe consequences for cardiology practices. Offenders may face a minimum five-year exclusion from federal programs via the HHS OIG list, halting participation in Medicare and Medicaid. Risks also arise in vendor deals, device purchases, and service contracts that might seem like referral incentives.
Failing to follow regulations in cardiac device billing brings costs far beyond initial fines. Practices face a range of issues that can threaten their stability and standing.
Fines are just the start. Non-compliance creates major revenue losses, legal risks, and workflow disruptions. Practices may need to refund years of improper claims, leading to cash flow problems that could force closure.
Exclusion from Medicare and Medicaid cuts off access to most cardiac patients, especially older ones with CIEDs. This penalty impacts not just the practice but also individual doctors and staff found at fault, potentially ending careers.
Reputational harm adds to financial strain. Public records of violations can hurt referrals, hiring, and patient trust, especially in close-knit medical circles where news spreads fast.
Compliance investigations drain resources. Staff must spend time on document reviews and remediation while still caring for patients, often lowering service quality and increasing turnover.
Traditional compliance tactics, like occasional training or manual reviews, can’t handle the pace and complexity of modern cardiac monitoring. They fall short in managing real-time data from multiple sources.
Manual record-keeping struggles with the constant data flow from CIEDs and RPM devices. A single patient can produce thousands of data points monthly, too much for paper or basic digital systems to track effectively.
Scattered data systems hide risks. Using multiple manufacturer portals means losing a full view of data, missing key events for billing, and creating gaps that audits can exploit.
Older programs often spot issues only after they happen, usually during audits. By then, practices face full penalties without a chance to fix errors in advance.
Schedule a demo to see how automated compliance tools can reduce risks in your cardiac RCM.
Billing codes for cardiac device monitoring are among the toughest in healthcare. Unlike standard procedures, remote monitoring requires detailed records of timeframes, device types, and clinical actions to match strict rules.
CPT codes 93298 and 93299 cover CIED monitoring, with 93298 for technical aspects and 93299 for professional review. They need proof of 30-day periods, device data, and physician input. Both parts demand precise documentation to pass audits.
Remote Physiological Monitoring codes, like 99453 and 99454, focus on patient data rather than device function. They require records of setup, education, and clinical management. Overlap between CIED and RPM codes must be managed to avoid double-billing.
Managing patients with multiple devices adds difficulty. Tracking billable events across different code sets for a patient with, say, a CRT-D and heart failure monitor, needs careful coordination to stay compliant.
Proving medical necessity is another hurdle. Each monitoring event must show clear clinical need and patient benefit, backed by records strong enough for audits yet manageable in daily work.
Billing frequency rules create further challenges. Most codes have strict limits on how often they can be used, and tracking these across many patients manually is nearly impossible. Errors here often lead to claim denials.
Cardiac device data has unique security needs beyond typical medical records. Its constant flow across platforms and vendors must be protected while staying accessible for urgent care.
Dealing with multiple manufacturers complicates security. Each has its own portal with different standards and controls. Practices must ensure HIPAA compliance across all while keeping systems connected for patient care.
Mobile access adds complexity. Clinicians need device data on phones for emergencies, requiring tight authentication and encryption to avoid delays in care.
Data moving between homes, servers, EHRs, and practices must stay secure at every step. Since transmissions often happen off-hours, automated monitoring is essential to spot and address threats instantly.
Detailed audit logs are a must for tracking data access and changes. For large practices, managing this volume of records demands advanced systems to store and retrieve data during reviews.
Vendor relationships in cardiac monitoring bring compliance risks under Stark Law and Anti-Kickback rules. Deals with tech providers or manufacturers can look like referral incentives if not structured carefully.
Free or low-cost services are a frequent issue. Discounts on monitoring tools or support from vendors might violate rules unless they reflect fair market value and have a clear, unrelated purpose.
Revenue-sharing deals need close attention. Paying practices based on patient volume for monitoring often conflicts with regulations unless it fits specific exceptions.
Marketing or educational materials from vendors can pose risks if they push specific devices or referrals. Practices must regularly review vendor contracts and financial ties while sticking to safe harbor rules.
Selecting vendors requires thorough checks beyond just money, looking at indirect benefits or support that might affect compliance. Strong compliance means ongoing training, audits, and following legal exceptions.
Effective compliance for cardiac billing needs a tech-driven approach that weaves rules into daily tasks, not as a separate chore. The best systems combine automation, regular training, and forward-thinking risk checks for continuous improvement.
Constant Monitoring and Audits
Modern systems track billing, documentation, and rule-following in real time. Algorithms catch potential issues early, allowing quick fixes before they become violations.
Good audit tools check multiple areas at once, like code accuracy, record completeness, and billing limits. Automation helps spot and fix errors instantly instead of waiting for external reviews.
Continuous tracking also shows trends that might point to bigger problems, like odd billing patterns signaling a need for updated training or processes.
In-Depth Staff Training
Regular training on Anti-Kickback rules, including exceptions and reporting, is required for all staff. Effective programs go further with role-specific lessons, real-world scenarios, and updates on new rules.
Training must tackle daily challenges. Technicians need to know documentation rules for codes, while admin staff learn billing limits. Clinicians should grasp both compliance and clinical reasons behind rules.
Cardiac billing is complex, needing specialized focus beyond general compliance. Staff must understand device details, data interpretation, and links between monitoring types and codes.
Forward-Looking Risk Checks
Assessing risks means reviewing operations to spot weak points before they cause issues. This covers vendor ties, billing habits, and documentation for potential regulatory problems.
These checks should account for cardiology’s unique needs, like multiple vendors, emergency demands, and complex patients. They must look at current gaps and future risks from rule or tech changes.
Legal protections exist for arrangements like in-office services and documented partnerships. Using these properly lets practices pursue useful deals while staying compliant.
The in-office services exception allows direct monitoring for patients if specific conditions are met. This can be helpful for RPM when practices supply devices and services themselves.
Fair market value deals offer a safe way to work with vendors. Paying standard rates for needed services, like monitoring access, usually fits within legal protections if unrelated to referrals.
Personal services exceptions can cover training or support from vendors if documented correctly. This helps with tech setup and education for monitoring.
Legal protections require strict adherence to detailed conditions for business deals. Success depends on knowing each rule’s specifics and shaping agreements to match every requirement.
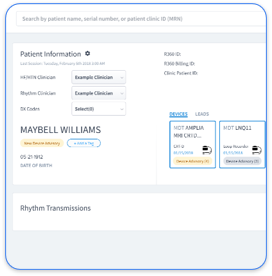
RhythmScience’s Rhythm360 platform helps cardiology practices tackle compliance challenges in cardiac device management. It integrates data from various sources and automates tasks to improve workflows and revenue while meeting regulatory needs.
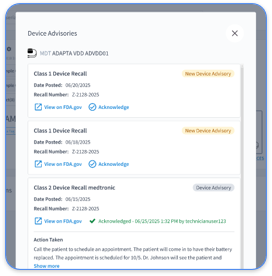
Rhythm360 identifies billable events and supports proper documentation for cardiac monitoring. It tracks CPT codes like 93298 and 93299 for CIEDs, ensuring periods and data meet standards. For RPM codes like 99453 and 99457, it aids records of activities, helping follow billing limits while boosting revenue.
With two-way EHR integration, Rhythm360 ensures smooth data transfer to practice systems, maintaining audit trails. Case studies from RhythmScience show practices gaining up to 300% more revenue through better code capture and efficiency.
Rhythm360 solves data fragmentation by unifying information from major manufacturers like Medtronic, Boston Scientific, Abbott, and Biotronik. This single view lowers risks from using multiple portals. It achieves over 99.9% data reliability with technologies like AI, ensuring access to critical details.
The platform meets HIPAA standards with strong security, including encryption and access controls for cardiac data. Its mobile app lets clinicians view patient info safely, balancing care needs with compliance.
Rhythm360 uses an AI alert system to highlight urgent events, cutting response times by up to 80%, based on RhythmScience data. This focuses clinicians on critical issues while ensuring documentation for audits. Automated reports and communication tools streamline tasks, keeping clear records of actions.
By cutting manual work, Rhythm360 boosts efficiency and reduces compliance risks, letting practices prioritize care. See how Rhythm360 can strengthen your practice’s compliance and cardiac RCM—schedule a demo now.
Even experienced cardiology practices can make critical errors in compliance, harming both regulation adherence and finances. Recognizing these pitfalls helps avoid costly missteps and build stronger systems.
Assuming One-Time Setup Covers All
Many practices think initial compliance steps last forever, not seeing that rules keep changing. Anti-Kickback and Stark Law issues remain key focus areas for healthcare in 2025, with shifting enforcement and guidance.
Agencies often update rules, offer new opinions, and adjust priorities based on industry trends. Ignoring these updates means finding gaps only during audits.
Cardiac monitoring tech evolves fast, with new devices and data methods bringing fresh compliance issues. Practices must regularly update programs to match current operations.
Overlooking Risks in Connected Systems
Advanced practices may downplay risks from using multiple tech vendors. Data moving across portals, EHRs, and billing tools creates vulnerabilities at every connection point.
Assuming each system’s compliance covers the whole setup misses risks in data handoffs or integrations. Gaps often appear where responsibilities aren’t clear.
Manual steps to link systems, like entering portal data into EHRs, lead to inconsistent records and audit issues, even if individual actions seem fine.
Neglecting People in Automation Plans
Tech-heavy practices sometimes roll out compliance tools without addressing staff needs. Resistance, poor training, or workflow clashes can weaken even top systems.
Effective automation needs change management, tackling staff concerns and ensuring tools fit daily tasks. Focusing only on tech often leads to failures that create new risks.
Assuming automation removes the need for oversight ignores the role of human judgment in handling exceptions. Even advanced systems need monitoring for unusual cases.
Seeing Compliance Only as Risk Avoidance
Many programs aim just to dodge fines, missing how compliance can improve efficiency and revenue. This narrow view skips chances for better operations.
Strong compliance systems built into workflows often enhance overall practice performance while cutting risks. Viewing compliance as a cost misses its broader value.
Good compliance offers benefits beyond protection, like better payer deals and efficiency. Practices excelling here often see higher reimbursements and stronger operations.
How does Rhythm360 handle CPT code compliance for remote monitoring?
Rhythm360 automates tracking of billable events for device and clinical activities, aligning with CPT codes like 93298 and 93299 for CIEDs and 99453 or 99457 for RPM. It monitors periods, checks data completeness, and matches documentation to rules, helping capture revenue while staying compliant.
What regulatory issues matter most for cardiology practices using remote monitoring?
Cardiology practices face several compliance challenges with remote monitoring. Accurate CPT coding and documentation are critical, requiring precise records of activities and intervals. HIPAA rules complicate managing data across multiple platforms and vendors. Stark Law and Anti-Kickback rules impact vendor deals and service contracts that might look like referral incentives. Proving medical necessity and respecting billing frequency limits add further complexity, especially with high-volume device data.
Can Rhythm360 assist with Stark Law and Anti-Kickback compliance?
Rhythm360 aids certain aspects of Stark Law and Anti-Kickback compliance with a vendor-neutral system integrating data from major manufacturers without bias. Its clear documentation and audit trails support compliance reviews. However, practices must still review contracts independently to meet legal exceptions, as Rhythm360 is a tech tool, not a substitute for legal advice.
Does Rhythm360 work for practices with devices from multiple manufacturers?
Rhythm360 handles multi-manufacturer setups by unifying data from companies like Medtronic, Boston Scientific, Abbott, and Biotronik into one system. This reduces risks from separate portals and inconsistent records, supporting billing compliance across device types.
How does automating RCM impact current compliance programs?
Automating RCM usually strengthens compliance programs with better monitoring and documentation. Tools like Rhythm360 fit into existing systems, offering real-time tracking and records to boost rule adherence. Success comes from blending automation with human oversight, ensuring staff focus on system monitoring and clinical judgment.
Regulations for cardiac device billing have grown from basic requirements into a vital part of strategy, distinguishing thriving practices from those facing inefficiencies and risks. Embracing this landscape offers lasting benefits beyond just avoiding fines.
Successful practices adopt automation for complexity, train staff thoroughly, use active monitoring, and see compliance as part of excellence, not a burden. These steps lead to better finances, patient results, and market position.
Enforcement is intensifying, and penalties for violations are climbing, with whistleblowers playing a bigger role. Sticking to manual or disjointed compliance methods increases risks that could threaten a practice’s future.
Tools like Rhythm360 help manage regulatory demands by consolidating data and automating tasks. This platform streamlines operations and revenue capture, letting practices focus on care while meeting standards.
Cardiology practices face a key decision: keep struggling with outdated compliance methods that drain efficiency, or adopt automation to integrate rules into strategy. Those making the shift will be better set for growth, quality care, and financial success.
Want to learn how Rhythm360 can enhance your cardiac RCM compliance? Schedule a demo today to explore how automation supports revenue and efficiency while aligning with regulations.