Last updated: February 24, 2026
Electrophysiology clinics now operate in a high-volume environment where global AF prevalence has doubled from 22.2 million in 1990 to 52.5 million in 2021. This surge places heavy pressure on CIED monitoring infrastructure. Fragmented OEM portals such as Medtronic CareLink, Abbott Merlin.net, and Boston Scientific LATITUDE force staff into manual, repetitive workflows. These fragmented processes increase operational costs and raise patient safety risks.
Security gaps appear when portable ECG devices transmit unencrypted user data over networks. CIED telemetry systems face similar exposure during data transmission. Administrative overload then compounds the risk through several recurring pain points:
HIPAA-compliant EP workflow automation now functions as a requirement rather than a convenience as 2025-2026 HIPAA Security Rule updates convert addressable safeguards into required controls, including MFA and encryption. Practices that move early on compliant automation gain measurable advantages in patient outcomes, operational efficiency, and financial performance.
EP clinics achieve reliable data security compliance by implementing seven focused HIPAA measures across their automated workflows.
1. HIPAA Risk Assessment for CIED Integrations
Clinics start by conducting a structured risk analysis that follows HHS OCR guidance for risk analysis. This process identifies vulnerabilities in multi-OEM data flows, EHR integrations, and telemetry transmission paths. Teams document threats to ePHI confidentiality, integrity, and availability across the entire CIED ecosystem.
2. Encryption for EP Clinic Data Transmission
Clinics protect CIED data by enforcing end-to-end encryption with HTTPS and TLS for all API traffic between OEM portals, automation platforms, and EHR systems. HTTPS/TLS encryption for all API communications secures data in transit, while encryption at rest protects stored CIED data and patient records.
3. Role-Based Access Controls in EP Workflows
Granular RBAC restricts access based on clinical roles. Electrophysiologists, device technicians, nurses, and administrative staff receive different permission sets that reflect their responsibilities. SMART on FHIR enables role-based access controls for granular permissions in multi-system setups.
4. Business Associate Agreements for EP Automation Vendors
Clinics execute comprehensive BAAs with every vendor that touches ePHI, including automation platforms, EHR vendors, and cloud providers. BAAs are mandatory with EHR vendors and third-party plug-in providers for interoperable EMR connections. These agreements define security expectations and breach responsibilities.
5. Automated Audit Trails for EP Compliance
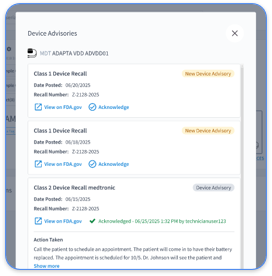
Robust logging tracks every access event involving CIED data, ICD alert responses, and EHR changes. FHIR Provenance and AuditEvent resources log all data access and modifications and create a defensible record for audits and investigations.
6. Multi-Factor Authentication for Mobile PHI Access
MFA protects all entry points, with special focus on mobile apps used for after-hours alert management. 2026 HIPAA updates mandate multi-factor authentication for all system access to ePHI. Clinics that implement MFA now align with these upcoming requirements.
7. AI Monitoring Aligned With 2026 Security Updates
AI-powered monitoring tools flag unusual access patterns and potential intrusions in real time. These tools must follow emerging AI governance standards while supporting HIPAA Security Rule updates scheduled for finalization in May 2026 that emphasize logging, monitoring, and incident response.
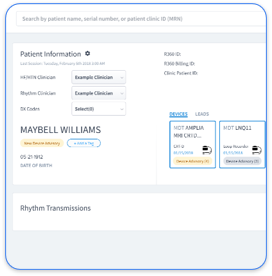
EP clinics protect CIED data more effectively when they standardize security across all OEM sources. Traditional setups require separate security protocols for each manufacturer portal, which creates inconsistent protections and heavy administrative work. Vendor-neutral platforms remove these silos and apply one security framework across every data source.
EHR integrations with Epic, Cerner, and Athenahealth require strict control of data transmission. HIPAA-compliant EMR systems require end-to-end encryption for data at rest and in transit. Bidirectional data flows must preserve integrity as information moves between CIED systems, automation platforms, and the EHR. API connections should use OAuth 2.0 authorization along with TLS encryption.
Rhythm360’s architecture supports these requirements through integrations that maintain more than 99.9% data transmissibility while enforcing strong security controls. The platform’s redundant data feeds keep connectivity active when OEM servers experience downtime. This design prevents delays in critical alerts. Unlike proprietary solutions from PaceMate or Implicity that tie clinics to specific vendor ecosystems, Rhythm360’s vendor-neutral model connects with any CIED manufacturer while keeping security standards consistent.
BAA management becomes simpler when clinics work with a single automation platform instead of separate agreements for each OEM vendor. This consolidation reduces legal overhead and keeps data protection standards uniform across all CIED data sources. Schedule a demo to see how vendor-neutral security architecture streamlines compliance management.
Rhythm360 brings HIPAA-compliant EP workflow automation into one secure platform that unifies CIED data from all major manufacturers. AI-powered triage tools cut critical alert response times by up to 80% and maintain complete audit trails for every access and clinical action. Clinics see faster clinical decisions, better outcomes, and stronger revenue capture through automated CPT documentation.

The mobile application extends this secure environment to after-hours care. MFA-protected access aligns with 2026 HIPAA requirements and keeps PHI protected on personal and shared devices. When a Saturday transmission reveals new-onset atrial fibrillation, clinicians can review the alert, coordinate anticoagulation, and document the intervention within a single HIPAA-compliant workflow that helps prevent stroke.
Revenue performance improves as Rhythm360 automates billing documentation and CPT capture for remote monitoring services. Many clinics report up to 300% revenue improvement from accurate, complete billing for codes 93298, 93299, and 99454. The platform removes manual transcription errors that cause denials and helps recover revenue that previously went unbilled.
Compared with competitors such as Murj or Octagos, Rhythm360’s vendor-neutral architecture offers greater flexibility and EP-focused functionality. The combination of electrophysiology-specific workflows and comprehensive HIPAA safeguards positions Rhythm360 as a strong choice for clinics that want both efficiency and regulatory confidence.
Successful EP workflow automation starts with a clear view of current operations. Clinics first evaluate OEM portal usage, staff time spent on manual tasks, and existing security controls. Teams then assess maturity across four areas: data integration complexity, alert management efficiency, billing documentation completeness, and HIPAA readiness.
Most implementations follow a 2 to 4 week roadmap. This period includes BAA execution with OEM vendors and cloud providers, configuration of integrations, and staff training. Common pitfalls include underestimating multi-OEM data normalization and providing limited training on new workflows. Dedicated project management and phased rollout plans help clinics maintain continuity of patient care.
Use this readiness checklist for compliant EP automation:
Schedule a demo to receive a tailored implementation roadmap that matches your clinic’s size, technology stack, and compliance goals.
Rhythm360 functions as a HIPAA-compliant platform that includes technical, administrative, and physical safeguards. These safeguards include encryption, role-based access controls, automated audit trails, and executed business associate agreements. The platform supports secure handling of PHI throughout EP workflows. Schedule a demo to review specific compliance controls.
Effective RBAC assigns different access levels to electrophysiologists, device technicians, nurses, and administrative staff. Electrophysiologists receive full clinical data access, while technicians focus on transmission review, nurses manage alert triage, and administrative users handle billing data. Each role receives only the minimum necessary permissions. Automatic session timeouts and detailed activity logs further protect CIED data.
Clinics begin risk assessment by mapping every data flow from OEM portals through automation platforms into the EHR. Teams identify vulnerabilities at each transmission point and review current security controls. They document threat scenarios specific to CIED telemetry, evaluate business associate relationships, and rank remediation tasks by risk level and regulatory impact. Regular follow-up assessments keep the program aligned with new technologies and threats.
EP clinics should use AES-256 encryption for data at rest and TLS 1.3 for data in transit. These standards protect API communications between OEM portals, automation platforms, and EHR systems. Database encryption, encrypted backups, and strong key management policies complete the protection for CIED data across its lifecycle.
BAAs define how each vendor protects PHI when multiple organizations handle CIED data. Every manufacturer, automation provider, cloud host, and integration partner must sign BAAs that describe security controls, breach notification timelines, and data use limits. These agreements create clear accountability and reduce regulatory risk while still allowing necessary data sharing for patient care.
Automated audit trails record every system login, data change, alert response, and clinical decision within EP workflows. These records demonstrate proper PHI handling during audits, support rapid breach detection, and guide incident investigations. They also provide data for quality improvement and show alignment with HIPAA Security Rule requirements for access monitoring.