Last updated: February 24, 2026
Standalone Software as a Medical Device (SaMD) analyzes CIED data independently, while Software in a Medical Device (SiMD) lives inside hardware like pacemakers, and both fall into FDA risk classes I to III based on clinical significance and patient condition severity. The FDA follows the International Medical Device Regulators Forum (IMDRF) risk framework, which categorizes software from lowest risk (Category I) to highest risk (Category IV) based on the criticality of the health condition and the impact of the software output on care decisions.
| Type | Definition | Cardiology Example | Risk Class |
|---|---|---|---|
| SaMD | Standalone software performing medical device functions | Dashboard analyzing AFib from CIED data | Class II/III |
| SiMD | Software integral to medical device hardware | Pacemaker firmware controlling pacing algorithms | Class II/III |
The regulatory pathway relies on premarket submissions, with 510(k) for moderate risk and PMA for high risk. It also requires Quality Management System compliance under 21 CFR Part 820 and alignment with standards such as IEC 62304 for software lifecycle processes and ISO 13485 for quality systems. Cardiology RPM platforms must show reliable data ingestion, AI-supported alert triage for clinical decision support, and clean integration with billing systems for CPT codes 93298 and 99454. FDA's January 2026 Clinical Decision Support Software Guidance outlines updated expectations for AI-enabled diagnostic and treatment recommendation systems in cardiovascular care.
SaMD FDA classification treats standalone software such as dashboards that diagnose AFib from CIED data as Class II or III devices that require premarket notification or approval based on clinical risk and intended use. The framework evaluates two factors: the healthcare situation or condition addressed, which can be critical, serious, or non-serious, and the significance of the SaMD information to healthcare decisions, which can treat or diagnose, drive clinical management, or inform clinical management.
| Risk Class | Healthcare Condition | Cardiology Example | Regulatory Controls |
|---|---|---|---|
| Class I | Non-serious conditions, informational | Fitness apps tracking heart rate trends | General controls, often 510(k) exempt |
| Class II | Serious conditions, clinical management | AI software recommending medication adjustments for stable hypertension | 510(k) premarket notification plus special controls |
| Class III | Critical conditions, treatment or diagnosis | AI identifying STEMI from ECG and triggering cath lab activation | PMA with clinical trial evidence |
Category IV SaMD in cardiology represents the highest risk tier, where software directly treats or diagnoses life-threatening conditions. These systems require extensive clinical validation and PMA pathways, as seen in recent FDA clearances for AI-enabled cardiac diagnostic platforms.
The SaMD and SiMD distinction shapes how CIED monitoring ecosystems are designed. SiMD covers embedded software inside implantable devices such as pacemakers and ICDs that manage pacing, detect arrhythmias, and run device diagnostics. SaMD covers external monitoring platforms that receive, analyze, and present CIED data to clinicians for decision-making. Both categories must satisfy FDA software validation requirements, yet they follow different regulatory routes based on hardware integration and clinical risk profiles.
IEC 62304 defines lifecycle processes for SaMD and SiMD development and maintenance, while FDA's 21 CFR Part 820 Quality System Regulation and ISO 13485 set broader quality management expectations across markets. Together, these standards create a complete framework for software validation and quality assurance over the full medical device lifecycle.
| Standard | Scope | Key Requirements | FDA Alignment |
|---|---|---|---|
| IEC 62304 | Medical device software lifecycle | Planning, requirements, design, verification, maintenance | Recognized consensus standard |
| 21 CFR 820 | US Quality System Regulation | Design controls, risk management, validation | Mandatory for US market |
| ISO 13485 | Quality management systems | International QMS framework | Harmonized with FDA QSR |
IEC 62304 applies to all medical device software, including SaMD and SiMD, and sets structured lifecycle processes such as software safety classification, architecture design, and Software of Unknown Provenance management. FDA's September 2025 Computer Software Assurance (CSA) guidance modernizes validation by emphasizing risk-based testing instead of purely prescriptive documentation, which aligns US practices more closely with ISO 13485:2016.
The main implementation difference appears in documentation structure. IEC 62304 calls for specific software description documents, requirements specifications, and verification and validation protocols. FDA reviews focus on traceability matrices and anomaly resolution that support 510(k) submissions under Part 820 design controls. Cardiology RPM platforms gain efficiency by adopting IEC 62304 processes, which satisfy FDA expectations and support international market access.
FDA's 2026 policy for device software functions requires SBOMs, clear vulnerability patching processes, and cybersecurity risk management for RPM platforms that handle sensitive cardiac data. Cybersecurity in Medical Devices: Quality Management System Considerations sets detailed security expectations for connected medical devices.
Key 2025 and 2026 updates include FDA's July 2025 final guidance on Predetermined Change Control Plans (PCCP) for AI-Enabled Device Software Functions, which supports iterative AI algorithm improvements while preserving safety and effectiveness. RPM platforms must apply threat modeling, penetration testing, and secure development practices to comply with Section 524B cybersecurity requirements. AI-powered alert triage systems also require added validation under these frameworks, especially for high-risk cardiac use cases where algorithm changes can affect patient safety.
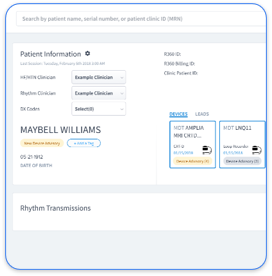
Rhythm360 delivers a vendor-neutral RPM platform that supports comprehensive CIED monitoring across all major device manufacturers. The platform reaches more than 99.9% data transmissibility through redundant data feeds, computer vision, and AI-driven data extrapolation, which removes the chaos of juggling separate OEM portals from Medtronic, Abbott, Boston Scientific, and Biotronik.

Core compliance and workflow features include:
A recent case highlights the clinical impact. On a Saturday morning, Rhythm360 AI flagged new-onset atrial fibrillation in a 72-year-old patient with a dual-chamber ICD. The automated alert notified the on-call electrophysiologist through a secure mobile app. The clinician started anticoagulation within hours and likely prevented a stroke. Without vendor-neutral monitoring, the arrhythmia might have gone unnoticed until the next routine transmission review.
Legacy solutions such as PaceMate or Implicity often retain OEM-specific constraints that limit visibility. Rhythm360's vendor-neutral architecture provides a unified view across device types and manufacturers. The SaaS pricing model scales with clinic size and usage, and typical implementations complete within days or weeks instead of the months often required for custom builds.
Schedule a demo to see how Rhythm360 converts fragmented CIED monitoring into streamlined, profitable RPM operations.
Successful FDA-compliant RPM deployment depends on a structured approach to regulatory, technical, and operational requirements.
The build versus buy decision shapes timelines and risk. Custom RPM development often requires 12 to 18 months for FDA validation and market clearance. Established SaaS platforms such as Rhythm360 usually support deployment within days to weeks. Total cost of ownership must include ongoing compliance maintenance, cybersecurity updates, and clinical validation, which proven platforms already manage.
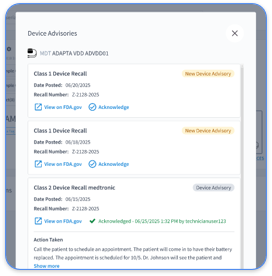
Failure to meet 2026 AI validation and cybersecurity requirements increases the risk of FDA audits and enforcement, while OEM data silos create blind spots for Class II and III cardiac events that demand rapid intervention. Frequent missteps include weak risk assessment documentation, limited cybersecurity testing, and poor integration planning that leaves critical data gaps.
The US SaMD market growth from $205.12 million in 2024 to a projected $715.00 million by 2033 shows rising adoption and tighter regulatory focus. FDA's November 2025 expanded guidance on AI and ML-based SaMD lifecycle management speeds approvals for adaptive algorithms while demanding more rigorous validation.
Practices must prepare for stronger cybersecurity rules that include multi-factor authentication, improved audit logging, and stricter access controls expected in final 2026 HIPAA updates. AI-powered clinical decision support systems also require additional validation under new FDA frameworks, especially for high-risk scenarios where algorithm changes can alter outcomes.
Rhythm360's CIED monitoring dashboard offers a clear SaMD example in cardiology. The platform independently analyzes data from pacemakers, ICDs, and loop recorders to detect arrhythmias, device malfunctions, and battery depletion. The software runs separately from the implanted hardware, receives transmitted data, and applies AI algorithms to flag clinically significant events that require physician review. The embedded software inside the devices remains SiMD.
FDA medical device software certification follows a risk-based pathway. Class I devices often qualify for 510(k) exemption with general controls only. Class II devices require 510(k) premarket notification that shows substantial equivalence to predicate devices plus special controls. Class III devices require PMA supported by clinical trial evidence. Each pathway needs software validation documentation aligned with IEC 62304, cybersecurity assessments, and quality system compliance under 21 CFR Part 820. Typical review timelines range from about 90 days for 510(k) to 180 days or more for PMA.
SaMD operates as standalone software that performs medical device functions, such as mobile apps that analyze ECG data or cloud platforms that monitor CIED transmissions. SiMD refers to software that is integral to medical device hardware, such as pacemaker firmware that controls pacing or ICD software that detects arrhythmias. Both require FDA compliance, yet SiMD follows the hardware device's regulatory pathway, while SaMD undergoes a separate software-specific evaluation based on clinical risk and intended use.
FDA software guidance for RPM platforms requires full lifecycle management that includes software safety classification, risk-based validation, and cybersecurity controls with SBOM documentation. Platforms must show secure data transmission, strong user authentication, vulnerability management, and detailed audit trails. AI-enabled features need added validation under the 2025 PCCP guidance, and clinical decision support functions must align with the January 2026 CDS guidance for diagnostic and treatment recommendation algorithms.
2026 cybersecurity expectations push RPM platforms to adopt threat modeling, penetration testing, static and dynamic code analysis, and structured vulnerability disclosure programs. Connected cardiac devices must provide SBOMs, support secure patching, and maintain documented cybersecurity risk management. Practices must apply vendor security updates quickly, use network segmentation, and maintain incident response procedures. Multi-factor authentication and stronger audit logging become mandatory for access to patient cardiac data, and HIPAA updates are likely to reinforce enforcement.
The 2026 FDA environment requires advanced SaMD and SiMD compliance for cardiology RPM programs to succeed. Practices that master these rules and adopt vendor-neutral platforms such as Rhythm360 remove OEM silos, cut alert fatigue, and recover lost revenue through accurate CPT billing. Schedule your demo today to secure FDA-aligned RPM while unlocking the 300% revenue potential of comprehensive cardiac remote monitoring.