November 14, 2025
Managing device and lead recalls can be a major challenge for busy clinics. Our latest Rhythm360 software upgrade takes the stress out of the process. We are saving your team countless hours and improving assurance that patients are protected. But not all platforms are created equal; some say they handle recalls, but do they really make it easier for your staff to track outreach and ensure compliance?
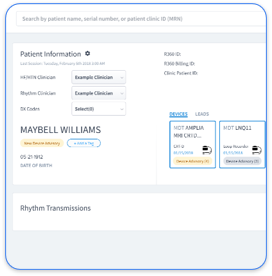
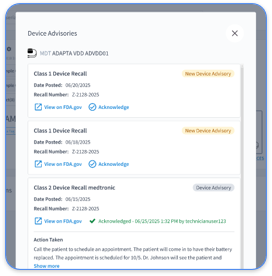
Rhythm360 has always supported recall and advisory management, and our new advancements make it even simpler. With enhanced advisory tagging technology, you get smarter longitudinal management for every recall.
If your clinic is ready for more effective recall management? Reach out for a complementary demo so we can show you how Rhythm360 streamlines your workflow and safeguards your patients with every upgrade.