Last updated: February 24, 2026
A HIPAA-compliant telehealth platform provides a signed Business Associate Agreement (BAA), end-to-end encryption for data in transit and at rest, secure authentication with multi-factor authentication (MFA), comprehensive audit logging, and secure U.S.-based data storage. 2025-2026 updates emphasize encryption by default, MFA as standard, and stronger access log monitoring, with AI-powered tools using fully de-identified patient data or operating under full HIPAA protection.
Essential compliance checklist for cardiology platforms:
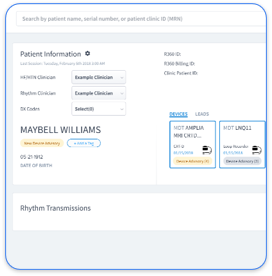
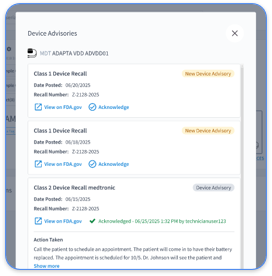
Rhythm360 focuses on cardiology practices that manage multiple cardiac implantable electronic devices (CIEDs) and chronic cardiac conditions. This vendor-neutral, HIPAA-compliant platform unifies data from Medtronic, Boston Scientific, Abbott, and Biotronik into a single dashboard with greater than 99.9% data transmissibility supported by redundant feeds, computer vision, and AI-powered extrapolation.
The AI-powered alert triage system reduces critical response times by 80% and filters non-actionable noise to prevent alert fatigue. Bi-directional EHR integrations with Epic, Cerner, and Athenahealth support automated CPT code capture for 93298, 93299, and 99454, which helps practices increase profitability by up to 300%. The secure mobile app allows cardiologists and electrophysiologists to review transmissions, sign reports, and coordinate care from any location.

Pros:
Cons:
Schedule a demo to see how Rhythm360 unifies device monitoring and increases RPM revenue.
Doxy.me offers a free HIPAA-compliant browser-based platform with virtual waiting rooms and custom branding on paid plans that range from $35 to $50 per month. The platform supports encrypted video sessions, patient queuing, and no PHI storage on servers, which works for basic cardiology consultations.
Pros:
Cons:
VSee is a purpose-built telehealth platform with safeguards such as encryption, role-based access, and BAAs. The platform supports secure video consultations, digital intake, and virtual waiting rooms, which suits specialty cardiology clinics that need custom workflows.
Pros:
Cons:
Zoom supports HIPAA compliance with a signed BAA and proper configuration on Zoom One Pro, Business, or Enterprise plans that start at $149.90 per year per license. The platform offers EHR integrations with Epic and Cerner, group visits, and AES 256-bit encryption.
Pros:
Cons:
Mend targets large clinics and health networks with HIPAA-compliant video, automated reminders, digital intake, secure messaging, and support for high visit volumes. The platform focuses on administrative efficiency rather than clinical monitoring or device data.
Pros:
Cons:
eCloudsHealth focuses on cardiology practices with intelligent digital check-in that captures cardiac histories, medications, and risk factors. The platform supports EMR integration via HL7, FHIR, or API and includes automated cardiovascular screening tools.
Pros:
Cons:
QT Medical’s ECGcloud platform supports remote 12-lead ECG testing via PCA 500 for telehealth and offers integration for real-time ECG access and chronic cardiovascular disease monitoring.
Pros:
Cons:
AliveCor delivers HIPAA-compliant patient engagement through KardiaMobile devices and the KardiaPro platform for ECG monitoring and biometrics with clinician review, which generates real-world evidence from ECG safety data.
Pros:
Cons:
| Platform | HIPAA Features | Cardiology Score | Best For |
|---|---|---|---|
| Rhythm360 | BAA, encryption, audit logs, mobile security | 10/10 | Vendor-neutral CIED and RPM integration |
| Doxy.me | BAA, encryption, virtual waiting rooms | 7/10 | Free basic telehealth consultations |
| VSee | BAA, encryption, role-based access | 8/10 | Custom cardiology workflows |
| Zoom Healthcare | BAA, AES 256 encryption, EHR integration | 7/10 | Small to medium clinic video calls |
Doxy.me provides the most robust free HIPAA-compliant telehealth option with browser-based access and virtual waiting rooms. Zoom’s free version is not HIPAA-compliant and requires paid plans that start at $149.90 annually for compliance. Free platforms support basic cardiology consultations but lack specialized RPM integration, automated billing, and multi-vendor device support that comprehensive cardiac care management requires.
Zoom can operate as a HIPAA-compliant telehealth solution when configured correctly with a signed BAA and a Pro, Business, or Enterprise plan. Compliance depends on encryption, access controls, audit logs, and correct admin configuration for PHI. Zoom’s free version is not compliant, and the platform does not provide specialized cardiac device monitoring or RPM billing automation that cardiology practices need.
2025-2026 HIPAA updates emphasize encryption by default for data in motion and at rest, MFA as standard, and stronger access log monitoring. AI-powered tools must use fully de-identified patient data or operate under full HIPAA safeguards. Cardiology practices should reassess BAAs to confirm that vendors meet updated security standards and update policies for telehealth protocols, especially those tied to RPM billing compliance.
Successful HIPAA-compliant telehealth implementation for cardiology follows a clear rollout plan:
Rhythm360 supports this process with streamlined onboarding, bi-directional EHR integration, and mobile alert setup that typically complete in days to weeks, which accelerates ROI.
Rhythm360 directly addresses the core challenges facing cardiology practices in 2026. Generic telehealth platforms support video consultations but cannot unify fragmented OEM data or automate complex RPM billing requirements. Rhythm360’s vendor-neutral CIED normalization technology processes data from Medtronic, Boston Scientific, Abbott, and Biotronik through a single interface, which removes the administrative burden of multiple portal logins.
The AI-powered alert triage system reshapes clinical workflows by cutting critical response times by 80% and filtering non-actionable notifications that drive alert fatigue. When a patient experiences new-onset AFib on a Saturday morning, Rhythm360 mobile alerts support immediate intervention that can prevent a stroke by Monday. This type of scenario shows how the platform influences outcomes rather than only improving back-office efficiency.
Automated CPT code capture also drives significant revenue gains. With the mobile cardiac telemetry market projected to reach $4 billion by 2035, practices using Rhythm360 capture revenue that previously went unbilled through accurate RPM documentation, which can increase profitability by up to 300%.
Schedule a demo to see how Rhythm360 reduces alert response times, eliminates data fragmentation, and maximizes RPM revenue for your cardiology practice.
Rhythm360 is the leading HIPAA-compliant telehealth platform for cardiology because it is built for cardiac care with vendor-neutral CIED integration, AI-powered alert triage, and automated RPM billing. Unlike generic platforms, Rhythm360 unifies data from all major device manufacturers and reduces critical response times by 80%.
Rhythm360 provides comprehensive HIPAA-compliant RPM services for CIED monitoring and chronic conditions such as heart failure and hypertension. The platform includes automated CPT code documentation for 93298, 93299, and 99454, which helps practices maximize revenue while maintaining full compliance.
Zoom can operate as a HIPAA-compliant platform with proper configuration and paid plans, but it does not offer specialized cardiac device integration, automated RPM billing, or AI-powered alert triage. Dedicated platforms such as Rhythm360 address complex cardiology workflows that extend beyond basic video visits.
Doxy.me provides a comprehensive free HIPAA-compliant telehealth platform with browser-based access, virtual waiting rooms, and encrypted video sessions. Free platforms, however, do not provide specialized cardiac monitoring, multi-vendor device integration, or automated billing features that cardiology practices need for full RPM programs.
Google Meet can be HIPAA-compliant through Google Workspace for Healthcare with a signed BAA and careful configuration. It still lacks cardiac device integration, automated RPM billing, and cardiology-focused workflow tools, so it functions as a generic telehealth option rather than a dedicated cardiac RPM solution.
Generic HIPAA-compliant platforms cover basic telehealth needs, but cardiology practices need specialized solutions that address OEM fragmentation, automate RPM billing, and prioritize critical alerts. With telecardiology projected to reach $63.46 billion by 2034, practices benefit from platforms designed for cardiac care complexity.
Rhythm360 delivers vendor-neutral CIED monitoring, AI-powered clinical workflows, and automated revenue capture in a single HIPAA-compliant platform. Schedule a Rhythm360 demo today to unify telehealth and RPM operations while improving patient outcomes and practice profitability.